 |
| https://en.wikipedia.org/wiki/Chemical_reaction |
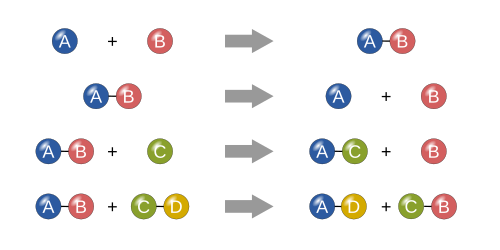
There are four different types of chemical reactions. These types of reactions are called 1. Synthesis, when two or more thing come together to create something bigger. 2, Diffusion, when a molecule splits into smaller pieces or individual atoms. 3, Single displacement, When there is a small molecule/atom and a molecule of 2 or more and one atom or piece of big molecule breaks out and switches places with the small piece. 4, Double displacement, When there are two "big" molecules and a piece of atom of each of them come of and join the other molecule like square dancing.
For S&EP we used models because we made chemical reactions, then got a worksheet that showed us the formula and had us chose which type of reaction it was. This brought me closer to mastery because now I understand how chemical reactions work. I think that now I would be able explain it to someone else who does not understand.
This week's XCC is Cause and Effect because we did chemical reactions, and that is when you mix these things together, something else happens.
