Dear National Science Foundation (NSF),
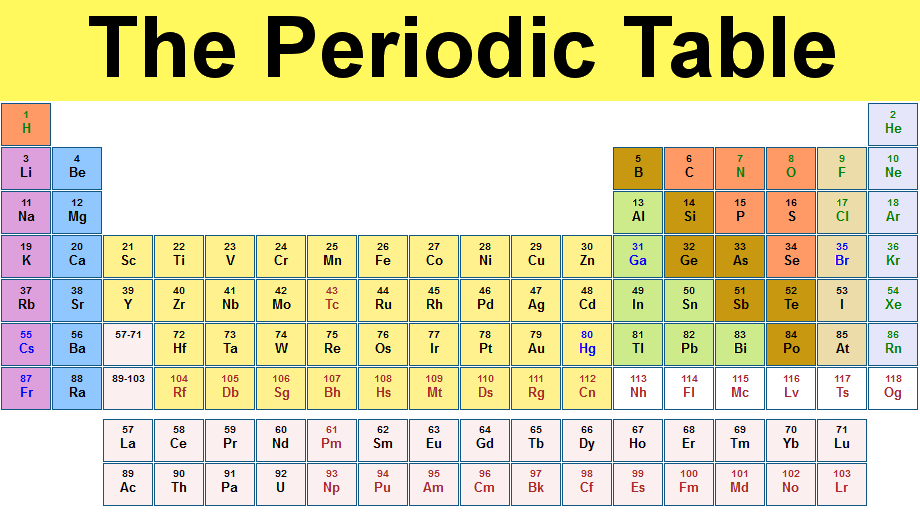
I am Dmitri Mendeleev's friend and I have some things to say about why he is not crazy. You may think he is crazy for leaving spaces, but he is leaving spaces for atoms that have not been discovered because (example) is there is a 30 and 33, why wouldn't there be a 31 and 32? He can predict its qualities because elements in the same column/family will have similar properties. If he doesn't leave spaces there will be no patterns to follow.
One property that he took in mind while making this table is reactions. When look at it, it looks like a messed up blob. But when you look at it more, you see that in the columns are elements that react the same. This is because (as you will discover later) they all have the same amount of electrons on their outer shell so that gives them the same reactions.
Another property he took into mind is atomic weight. This helps by making things easy to find. This also shows that things react because how many electrons are in the outer shell. That affects electrons in outer shell because for every proton, there is a neutron.
He is also right because people discovered the elements that he left spaces for. Those elements fit perfectly it the gaps and reacted like he expected them to. This is again, because of the electrons in the outer shell. His idea actually makes so much sense and is so logical that some people also thought of it.
So in conclusion, what may seem like a complete mess has a method, and that method is pretty logical. So you should not shut him down. He has created something amazing, that will help you define how elements work.
Tuesday, September 19, 2017
Friday, September 15, 2017
Weekly Blog (9/15/17) Periodic table
 |
| https://www.chemicool.com/ |
For S&EP we used mathematics to find out that the different 8 columns have different electrons in their outer shell. Such as in the first row, the have 1 electron in their outer shell so they all react the same. And the second row have 2 valence electrons etc.. This brought me closer to mastery because now I understand why they react the same.
This week I was a creator because I was answering a lot of people questions and helping them out.
Wednesday, September 6, 2017
Weekly blog (9/8/17) Isotopes and Ions
 |
| https://socratic.org/questions/what-is-the-difference-between-atom-and-molecule |
This week in class we learned all about Isotopes and Ions. An Ion is when there are uneven amount of electrons-protons. An Isotope is when there are uneven amounts of Protons and Neutrons.
This week for S&EP we used mathematics to find out if something was an Ion/Isotope. This brought me closer to mastery because now I understand what Ions/Isotopes are.
I believe that this week I was a learned because I really like chemistry and I want to know about it.
Letter to King Arthur
Dear King Arthur,
My findings say that Crown 4 (Found by Gawain) is the golden crown. I know that crown four is the gold crown because it has the same density as gold. Density is how much stuff in is a given space. A mathematical equation to find density is grams/cm3.
I found that Crown 4 is the golden crown by using the equation grams/cm3 on all of the crowns. My team worked to together so that 2 of us did two crowns and 1 of us did 1 crown. Then after we had all the density numbers, we compared them to the densities that Merlin supplied us.
I also found that crown 1 (Found by Lancelot) was silver, Crown 2 (Found by percival) was Aluminum, Crown 3 (Found by Bedivere) was steel, Crown 5 (Found by Galahad) was Lead. I found all this using Merlin's density chart.
My findings say that Crown 4 (Found by Gawain) is the golden crown. I know that crown four is the gold crown because it has the same density as gold. Density is how much stuff in is a given space. A mathematical equation to find density is grams/cm3.
I found that Crown 4 is the golden crown by using the equation grams/cm3 on all of the crowns. My team worked to together so that 2 of us did two crowns and 1 of us did 1 crown. Then after we had all the density numbers, we compared them to the densities that Merlin supplied us.
I also found that crown 1 (Found by Lancelot) was silver, Crown 2 (Found by percival) was Aluminum, Crown 3 (Found by Bedivere) was steel, Crown 5 (Found by Galahad) was Lead. I found all this using Merlin's density chart.
Subscribe to:
Comments (Atom)